A press release on the issue of CBD manufacturers claiming no THC when detectable THC is still present in their products and what Folium Biosciences is doing to ensure a 0.0% THC product.
Woman Arrested at Disney World for CBD Product. Make Sure You Know Your CBD Manufacturer.
Colorado Springs, CO, May 14, 2019 – Recently, a 69-year-old woman from North Carolina was arrested at Disney World because she had CBD oil in her purse. She spent 12 hours behind bars before being released on a $2,000 bond. This CBD oil, which was recommended by her doctor, and was labeled as “0mg THC,” still prompting an arrest, according to WOFL Fox 35 Television in Orlando.
Q1 hedge fund letters, conference, scoops etc
The charge was later dropped, but the Orange County Sheriff’s Office said its deputy was following the law. While officials have said some forms of CBD are legal in Florida, the key issue here is that some CBD manufacturers may claim their product is “zero” THC, but police and employers’ tests and testing methodologies may show otherwise.
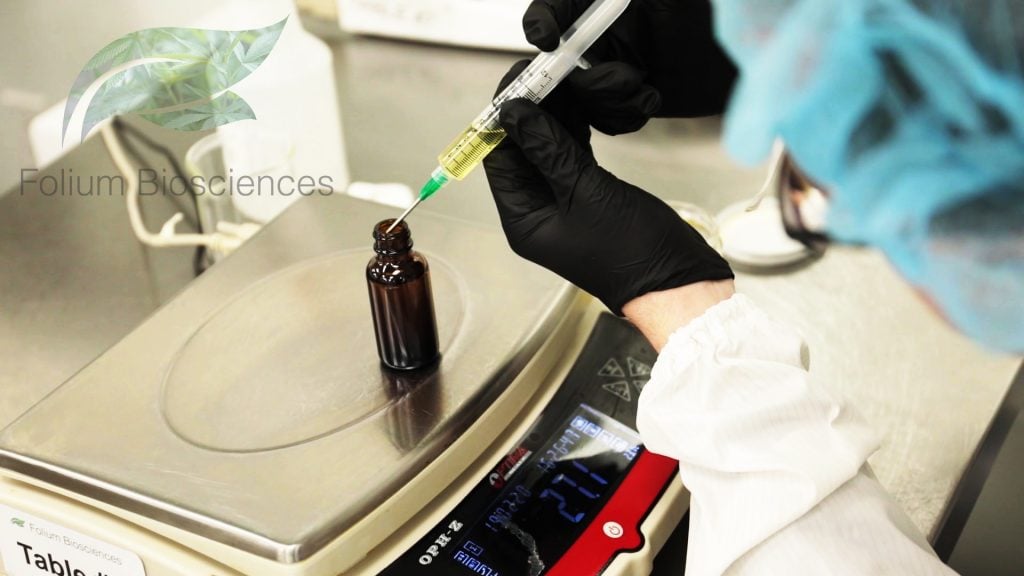
“This arrest brings up an important discussion. Even though some CBD manufacturers print on their label representing their product as no THC, unless they have the know-how, the technology, the resources and proprietary processing, their claims may not be completely accurate. We at Folium Biosciences have developed a reliable CBD/Hemp oil with a certified 0.0% THC content through our proprietary manufacturing process. We use a rigorous scientific-based quality process and protocols, routed in chemistry and physics, to ensure that our products are always “0.0% THC”,” said Folium Biosciences CEO, Kashif Shan and General Counsel Craig A. Brand, Esq. “It is long past time that law enforcement understands the molecules contained in Industrial Hemp products, and learns how to properly test and utilize the proper testing for the omission of THC. Off-the-shelf THC kits merely pick up the residual of any cannabinoid molecule regardless of whether or not the actual presence of THC exists; thus causing unnecessary and wrongful arrests”, says Craig A. Brand, Esq.
Folium Biosciences has established itself as the premier “0.0% THC” source of CBD liquids, powders, water and/or oil soluble products, cosmetics, pharmaceutical ingredients, supplements, etc… Folium Biosciences’ proprietary chromatographic purification process is able to selectively remove the psychoactive component, Delta 9-THC, from the Phytocannabinoid-Rich (PCR) hemp oil as well as other molecules, fats, and impurities selected for removal. The absence of detectable THC is confirmed through Folium’s state-of-the-art High Performance Liquid Chromatography (HPLC) instruments and laboratory testing equipment. Since Folium Biosciences’ purification process can selectively remove Delta 9-THC, while leaving intact the other synergistic molecules, terpenes and flavonoids in the refined oil, the result proves a clean, processed, refined non-psychoactive product. These resulting products still deliver all of the benefits of the remaining entourage effect for biological activity and efficacy. Important factors when looking at the Folium products’ solubility and absorbability, qualities which should at the top of all consumers. Folium Biosciences has been certified by the Colorado Department of Public Health and Environment (CDPHE) as a producer of THC-Free (non-detectable THC) Phyto-cannabinoid-Rich hemp oil through its State Certificate of Free Sale and all CDPHE inspections. Colorado has passed the Hemp Food & Cosmetic Act and has issued Folium Biosciences a Processing and Manufacturing License for its food and cosmetic products. Every Folium Biosciences order is backed by a Certificate of Analysis after a rigorous Quality Assurance check from its onsite internal laboratory(s) and Operating Procedures. Ongoing monitoring of representative batches through third-party labs is also performed regularly to ensure the quality and accuracy of the internal labs and the equipment therein. Folium Biosciences newest location is FDA registered, the company holds amongst its array of licenses, GMP, GPP, Manufacturing, Processing, Distribution and Pharmaceutical Active Ingredient licenses.
Folium Biosciences is headquartered in Colorado Springs. They are the largest vertically-integrated producer, manufacturer, and global distributor of hemp derived phytocannabinoids. Folium is a B2B, bulk and wholesale supplier of hemp derived CBD 0.0% THC oil, CBD water-soluble and nano-technology, CBD 0.0% THC edibles, cosmeceuticals, active lifestyle, and phytocannabinoids for animal health. Folium Biosciences continues to develop rare cannabinoid extraction and product formulation.
Potential B2B partners wanting to learn about Folium Biosciences revolutionary products should visit www.foliumbiosciences.com/order.