Muddy Waters is short Chinook Therapeutics Inc (NASDAQ:KDNY) because we believe it is highly unlikely that atrasentan, its lead product candidate, will be approved by the FDA. We conclude that atrasentan is inefficacious for chronic kidney disease.
We also conclude that atrasentan has been shown to be harmful to patients’ cardiovascular health. AbbVie and Chinook seem to have systemically manipulated research findings and presentation on atrasentan to obscure these trial results.
Even if atrasentan were efficacious and safe, it would be unlikely to gain approval because a competing drug, sparsentan, has received accelerated and exclusive orphan drug approval by the US FDA for IgA nephropathy, the condition targeted in Chinook’s only Phase 3 trial, the ALIGN study.
From trial data, atrasentan appears inefficacious for chronic kidney disease. In the largest available study, atrasentan had no statistically-significant effect on advancement to end-stage renal disease, showing only a minor numerical difference between drug and placebo groups.
All differences in UACR and systolic blood pressure between atrasentan and placebo patients disappeared after trial cessation, which suggests there was no actual improvement in the rate of renal decline. Scientific literature responding to SONAR also notes that the drug’s reported effects on UACR may be implausibly high.
Had the RADAR and SONAR trial periods fully reported hematocrit, we believe they would have shown that atrasentan’s primary effect was blood dilution – i.e., a hemodynamic effect. This probable hemodynamic effect was not only likely responsible for the temporarily improved biomarkers observed during the trial, but also would pose serious health risks.
Atrasentan is also harmful to patients’ health, especially older and more fragile patients such as those with diabetic kidney disease, the largest potential patient population. The numerical difference in heart failure between atrasentan and placebo was greater than the numerical difference in patients’ progression to end-stage renal disease.
Acute kidney injury was higher in the atrasentan group than in the placebo group. SONAR data reflects an increase in brain natriuretic peptide in patients treated with atrasentan in SONAR. BNP was normal for all patients entering treatment, but quickly became elevated in atrasentan patients.
Atrasentan is also associated with a statistically-significantly elevated rate of anemia. Anemia is a serious risk factor for disease progression in IgAN, an important patient population for Chinook. Reported mortality on atrasentan was numerically higher than reported mortality on placebo in the double-blind period of the SONAR trial.
This mortality increase in the atrasentan group is also present in the enrichment period. Avosentan, a drug with a similar mechanism to atrasentan, had a clinical trial abruptly stopped because of an increased risk of heart failure.
Studies done on atrasentan and similar drugs show that they raise heart failure risk. A sum of cardiac events from the FDA reported adverse effects gives 136 heart failure events on atrasentan and 93 on placebo.
A very small study using an arguably superior biomarker, inulin, to measure GFR, found that atrasentan seemingly made GFR worse. As discussed infra, sparsentan, which has exclusive orphan drug approval for reducing proteinuria in IgAN, is likely safer, showing a less alarming effect on heart failure and anemia.
Both AbbVie and Chinook appear to have manipulated facts and figures to conceal dangers associated with atrasentan. AbbVie appears to have manipulated graphs to modify the apparent effect of atrasentan by visually distorting enrichment and post-treatment periods, thereby modifying the slope of the drug effect.
AbbVie used composite endpoints in its data that conflated positive and negative outcomes from atrasentan, which runs contrary to best clinical practice. The SONAR authors then label these endpoints in a misleading way and include questionable endpoints in composites, including non-fatal stroke figures that entirely reverse the meaning of critical endpoints.
Chinook appears to have misled investors about the number of patients in which atrasentan was trialed, likely to give a false impression of drug safety. Chinook has also misrepresented atrasentan’s safety profile by claiming no SAEs associated with it or evidence of significant fluid retention, neither of which is accurate.
Chinook has misleadingly presented data by suggesting that hemodynamic factors played no role in the UACR results it showed. When Chinook reported on this study, it misleadingly gave percentages for patient benefit and adverse effect rates drawn from different sub-groupings of the sample.
The only placebo-matched data on atrasentan from Chinook has been delayed by request of the FDA, so there is no reason to expect that the safety issues supra have been overcome.
Introduction: On Study Methodology
Despite being designed to reduce the odds of serious adverse effects, the SONAR trial was abruptly halted. We see it as telling that despite the design, the data indicates increased risk of serious cardiovascular events from atrasentan.
SONAR made use of an enrichment-based study design, in which patients were stratified into “responder” and “non-responder” drug populations following a six-week “enrichment” period on atrasentan.
Patients were excluded from consideration if they had a previous hospital admission for heart failure, a history of pulmonary hypertension, any known non-diabetic kidney disease, a history of severe peripheral or facial oedema, or other-than-normal levels of brain natriuretic peptide (among other criteria).
Patients were required to enter treatment with an angiotensin receptor blocker or an angiotensinconverting enzyme inhibitor and a diuretic, both of which measures were intended to make heart failure less likely and renal repair more likely. Then, all eligible patients were given a sixweek round of atrasentan, after which they were stratified into responder and non-responder populations.
Responders were those patients who showed a 30% reduction in urine albuminto- creatinine ratio, who did not have substantial fluid retention, and did not show an increase in serum creatinine during the enrichment period—again, all measures intended to ensure that the population that continued in the trial would be as likely as possible to benefit from the treatment, and as unlikely as possible to suffer cardiovascular complications.
Only 3,666 patients—the responder population and about 1,000 non-responders—entered the double-blind treatment period, and only 2,648 patients completed the study. As we note supra, the trial was then abruptly halted.
Atrasentan: No Structural Repair
We conclude that the trial data showed that atrasentan is inefficacious for chronic kidney disease.
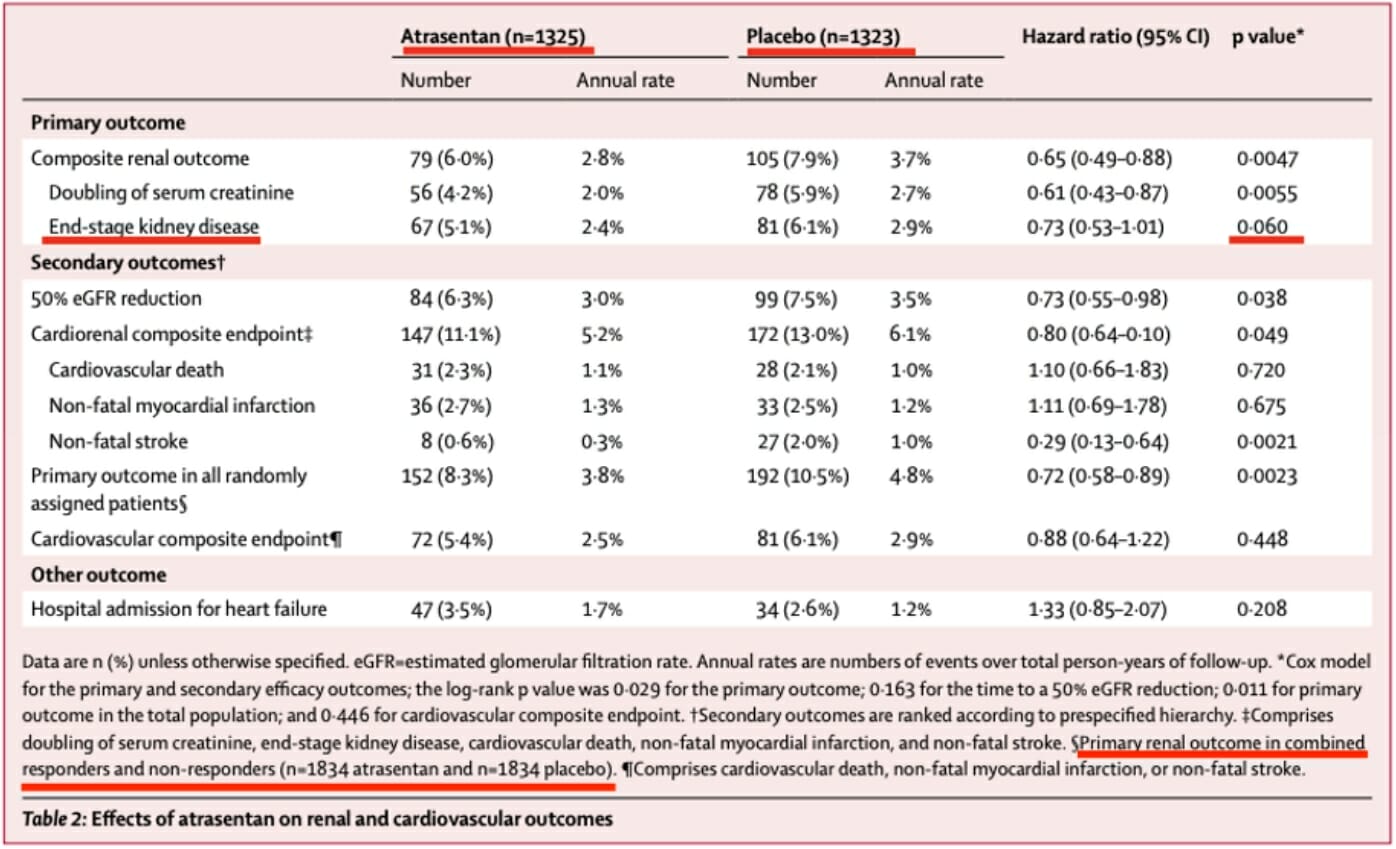
The study data shows that there was no statistically significant improvement in advancement to end-stage renal disease in the atrasentan versus the placebo cohort, only a numerical difference of 14 patients and a p-value of 0.06 with a percentage difference of 5.1% to 6.1% of patients.
After cessation of treatment, measurements of the surrogate biomarkers converged between the treatment and placebo arms. Systolic blood pressure converged before the treatment period was complete.
Experts involved in the atrasentan trials with whom we spoke explained that the effect was “functional”, not “structural”, maintaining that, in their opinions, there was some possibility of a structural effect becoming evident in the future.
No Statistically Significant Improvement in Advancement to End-Stage Renal Disease
Atrasentan’s direct effect on rates of advancement to end-stage kidney disease, as the below table taken from the SONAR analysis shows, does not reach statistical significance. It is only by conflating end-stage kidney disease with other renal endpoints, a technique we will discuss infra, that renal outcomes can be made to appear significant.
Difference in end-stage kidney disease, in the below table from the SONAR study, achieved a p-value of 0.06. The numerical difference in advancement to end-stage renal disease between atrasentan and placebo groups among responders was only 14 patients, a figure overshadowed by the excess cardiovascular events experienced by patients in the treatment arm of SONAR.
The numbers reported to the FDA reflect an even smaller between-groups difference of 8 patients, with a lower total number of events reported; it’s not obvious why the SONAR authors give different ESKD numbers in different places.
Read the full report here by Muddy Waters