The chemical element iron is one of the most abundant elements in the universe next to hydrogen, carbon, oxygen and helium. It can be commonly found in gaseous form inside stars such as the sun and in condensed form because it is a big part of many planets’ cores, such as Earth. However, scientists have had difficulties tracking interstellar iron, which appears to be invisible even to the most advanced equipment, and now they might know why.
In collaboration with the W.M. Keck Foundation, cosmochemists at Arizona State University found that the missing iron may not be much of a mystery after all. They believe the abundant iron in the universe bound with carbon molecules to form molecular chains known as iron pseudocarbynes. Their work was published in The Astrophysical Journal.
“We are proposing a new class of molecules that are likely to be widespread in the interstellar medium,” ASU Research Associate Professor Pilarasetty Tarakeshwar said in a statement.
They found this after examining clusters which contain only some metallic iron and learned that it might join with chains of carbon molecules to form new molecules. The team observed this after evidence obtained from stardust and meteorites suggested that there are many clusters of iron atoms in the universe.
Deep in space where interstellar iron is exposed to frigid temperatures, it acts as deep-freeze particles which allow chains of carbon molecules to bind with them.
“We calculated what the spectra of these molecules would look like, and we found that they have spectroscopic signatures nearly identical to carbon-chain molecules without any iron.” Tarakeshwar said, adding that because of this, “Previous astrophysical observations could have overlooked these carbon-plus-iron molecules.”
Because of these chemical reactions, scientists think interstellar iron isn’t really missing, but rather, masked in carbon-chain molecules that are more than common in interstellar space, hiding it from plain view.This research will also help scientists learn more about chain carbon molecules.
For example, scientists learned that carbon chains consisting of more than nine atoms are unstable. However, in interstellar space, there are more complex carbon molecules. It’s still a mystery how these chains are built.
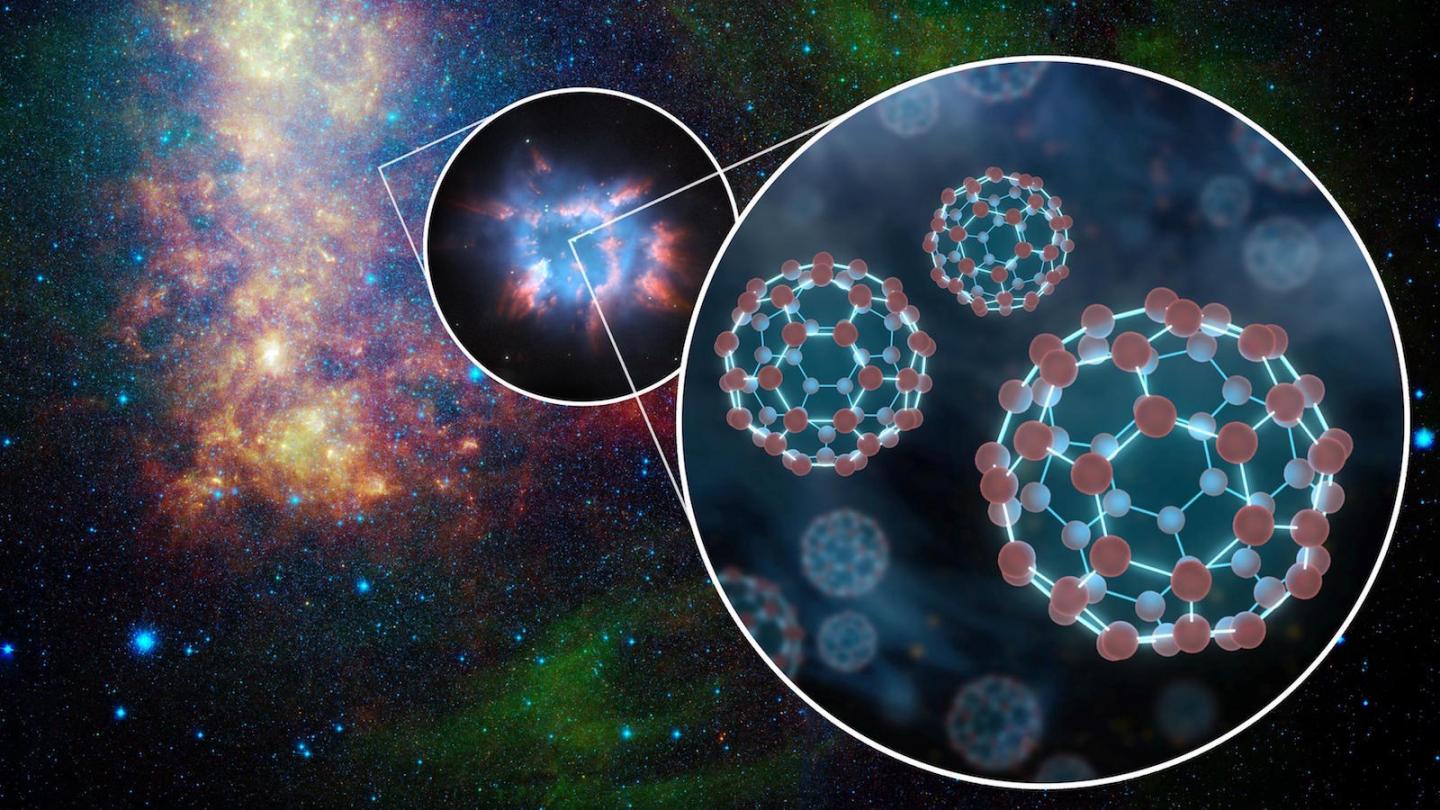
“Our work provides new insights into bridging the yawning gap between molecules containing nine or fewer carbonatoms and complex molecules such as C60 buckminsterfullerene, better known as ‘buckyballs,'” Frank Timmes of ASU’s School of Earth and Space Exploration said.